What Does a Salt Bridge Do Apex
SALT BRIDGE meaning - SALT BRIDGE definition - SALT BRIDGE explanati. This bridge contains an aqueous solution of sodium sulfate.

What Is The Purpose Of Salt Bridge Quora
A salt bridge is a device used in an electrochemical cell for connecting its oxidation and reduction half cells wherein a weak electrolyte is used.

. They contribute to protein structure and to the specificity of interaction of proteins with other biomolecules but in doing so they need not necessarily increase a proteins free energy of unfolding. What is the function of a salt-bridge in an electrochemical cell. If no salt bridge were present the solution in one-half cell would accumulate a negative charge and the solution in the other half cell would.
The purpose of a salt bridge is not to move electrons from the electrolyte rather its to maintain charge balance because the electrons are moving from one-half cell to the other. The salt bridge allows for ions to flow between the solution with the cathode and the solution with the anode. The salt bridge allows for ion transfer between the anode solution and the cathode solution.
It is a most commonly observed contribution to the stability to the. Its purpose is to keep the electrochemical reaction from. Apex Salinity Probe conversaion to ppm.
I like to keep my Salinity right around 1025 to 1026 range. Maybe I have been lucky bc I have never had any issues what so every with the Apex Salinity probe. What does a salt bridge do.
What does SALT BRIDGE mean. What does a salt bridge do. So it maintains both solutions at a neutral state meaning the electron flow will be able to continue for a more extended period of time.
What Does Salt Bridge Mean. As in the picture cations go to the right cell and anions go to the left. I think I understand what we dont want the salt bridge to do --we dont want it to react and that its function is to neutralize the solution so anions or cations dont build up and the cell stops.
Salt bridge is to. The function of the salt bridge is to allow the movement of ions from one solution to other without mixing of the two solutionsThuswhereas electrons flow in the outer circuit in the wire the inner circuit is completed by the flow of ions from one solution to the other through the salt bridgeMoreoverit helps to maintain the electrical neutrality of the solution in the two half cells. The purpose of a salt bridge is not to move electrons from the electrolyte rather its to maintain charge balance because the electrons are moving from one-half cell to the other.
Electrons move from the anode to the cathode leaving cations behind. Convert grams of reactant to moles of reactant using the molar mass of the reactant. The electrons flow from the anode to the cathode.
I can understand that anions go the left because the. The salt bridge usually consists of a strong electrolyte which is further made up of ions. The salt bridge allows for the flow of charge between vessels while keeping the cations seperated.
The salt bridge allows cations to move in the galvanic cell. How do you find average atomic mass from isotopic abundance. In order to keep the solutions electrically neutral an electrochemical salt bridge is suspended between the solutions.
I want to know how we know which salt to use for salt bridge. Updated on July 03 2019. In chemistry a salt bridge is a combination of two non-covalent interactions.
A salt bridge is a connection containing a weak electrolyte between the oxidation and reduction half-cells in a galvanic cell eg voltaic cell Daniell cell. Your problem is that through wires electrons move from one compartment to another but if theres no salt bridge and charge cannot build up current simply does not flow. Clearing a water softener salt bridge is just a matter of breaking up the dome of salt that has been formed in a water softeners salt tank creating a void between the water in the bottom of the salt tank and the salt that the water softener needs to properly regenerate its water softening resin.
Learn vocabulary terms and more with flashcards games and other study tools. Hydrogen bonding and ionic bonding. See below for the conversion chart.
Convert moles of product to grams of product using the molar mass of the product. Start studying APEX sem 1 chemistry. Ion pairing is one of the most important noncovalent forces in chemistry in biological systems in different materials and in many applications such as ion pair chromatography.
A salt bridge facilitates corrosion because corrosive reactions typically occur in the presence of electrochemical cells. Its not a homework question but I didnt know where else to ask. Postby Chem_Mod Sun Oct 02 2011 942 am.
In other words a salt bridge is a junction that connects the anodic and cathodic compartments in a cell or electrolytic solution. Imagine there was no separation between electrolyte as in the absence of the salt bridge there could result in the preferential reduction of one cation over the other. A salt bridge or ion bridge in electrochemistry is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell a type of electrochemical cell.
The electrons flow from the anode to the cathode. Convert moles of reactant to moles of product using the coefficients. Electrons flow from the anode to the cathode and as this happens the cathode solution gets more negatively charged as the cathode species are reduced and the anode solution gets more positively charged as the anode species are oxidized.
A salt bridge refers to a device used to form an electrochemical cell by providing a means to support the free flow of ions between the oxidation and reduction half-cell components. It maintains electrical neutrality within the internal circuit. Salt bridges in proteins are bonds between oppositely charged residues that are sufficiently close to each other to experience electrostatic attraction.
My reading on Apex salinity probe is 515 which roughly equates to 1025 specific.
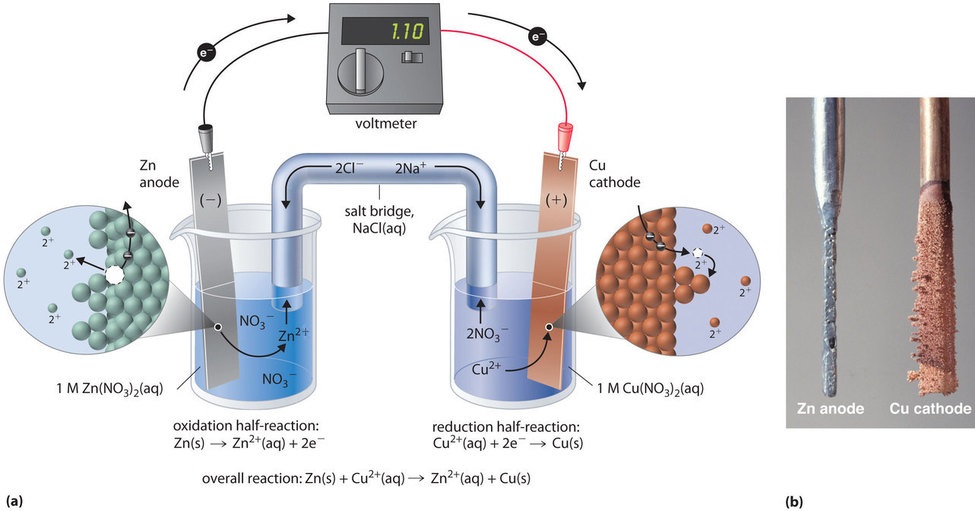
19 3 Voltaic Or Galvanic Cells Generating Electricity From Spontaneous Chemical Reactions Chemistry Libretexts

Galvanic Cell Animation Zinc Silver Chemistry Lessons Teaching Chemistry Chemistry Experiments

Salt Bridge Easy Science Electrochemistry Bridge Game Ap Chemistry
What Is The Function Of A Salt Bridge And How Are They Built Quora

Pin By Akila Vedam On Aki Train Journey Train Journey

Introduction To Electrochemistry I

What Is A Galvanic Cell Galvanic Cell Electrochemical Cell Science Clipart
What Is The Function Of A Salt Bridge And How Are They Built Quora

Electrochemistry Is There Always A Need For A Salt Bridge To Let A Galvanic Cell Work Continuously Chemistry Stack Exchange
What Is The Function Of A Salt Bridge And How Are They Built Quora

Electrochemistry Describe The Function Of A Salt Bridge What Would Happen If The Half Cell Compartments Of A Galvanic Cell Were Not Connected Chemistry Stack Exchange

Bipolar Behaviour Of Salt Bridges A Combined Theoretical And Crystallographic Study New Journal Of Chemistry Rsc Publishing Doi 10 1039 C8nj02194e

What Is The Purpose Of Salt Bridge Quora
What Is The Purpose Of Salt Bridge Quora

Electrochemistry Is There Always A Need For A Salt Bridge To Let A Galvanic Cell Work Continuously Chemistry Stack Exchange
What Is The Purpose Of Salt Bridge Quora
What Is The Function Of A Salt Bridge And How Are They Built Quora
Comments
Post a Comment